Hot issues
The FDA wants to ensure that domestic and foreign companies receive the same level of regulatory oversight.
Catch up on research PSM released about medicines imported from unregistered sources.
What if your blood thinners were made in an unverified location in Colombia? In March 2025, dozens of pharmaceutical shipments entered the U.S. that were manufactured in places no one would expect.
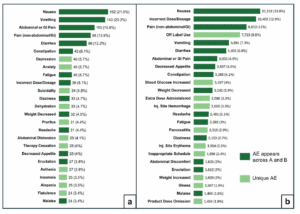
New research shows a correlation between compounded weight loss and diabetes injectables and increased adverse events. GIlead Sciences is suing another New York City pharmacy that allegedly sold counterfeit HIV medicine.
The FDA has issued a warning to the operators of the online pharmacy www.thesafepills.org for illegally selling unapproved and misbranded opioid medications, including tapentadol products marketed as Aspadol and Typendol. These unauthorized drugs, sold without a prescription, pose significant health risks such as overdose, addiction, and even death.
One of SafeChain’s three owners pleaded guilty. The company collected secondhand HIV medicines from patients and resold them to licensed U.S. pharmacies, forging paperwork to make them look legitimate.
A Kentucky doctor treated weight loss patients with research chemicals; CBP seized 90,000 alprazolam pills being smuggled into the U.S.
The FDA and Novo Nordisk are warning the public about counterfeit Ozempic injections circulating in the U.S. drug supply chain. The falsified products, labeled with lot number PAR0362 and serial numbers beginning with 51746517, were seized by the FDA on April 9, 2025. Their contents and safety are unverified and pose serious health risks.
Our podcast covers the latest in pharma crime and medicine safety.
Like your information on video? Subscribe to our YouTube playlist!

DrXanax's empire spanned years and nearly every U.S. state.

What have we learned about last year's fake Botox outbreak?

Click the images below to see more recent videos.